This fun experiment came from Roger Weller, geology instructor at Cochise College in Arizona, USA. If you can find it here in Guatemala, this is a great material for teaching students because the chemical is
safe (it is very bitter, so who would want to eat it?). It's also not bad for your teeth like sugar crystals! It grows nicely
either by the evaporation method or by the supersaturated method and it produces
nice crystals. The chemical is relatively inexpensive. It used to be
commonly found in grocery stores by the canning supplies because it was used in
making certain types of pickles. More recently, I have found it in the
spice section in the grocery stores.
Ammonium alum is used as an astringent and as a septic
pen for stopping bleeding when you cut yourself while shaving.
The actual chemical name of alum is "ammonium aluminum sulfate"
NH4Al(SO4)2.12H20

The name alum is also applied to other aluminum sulfates, potassium alum
and
chrome (chromium) alum. Chrome alum produces dark purple crystals, but it
can mixed with either ammonium alum or potassium alum to produce light purple
colored crystals. The dark purple crystals are unstable and eventually dry
out and decompose. The lighter colored mixtures are more stable.

chrome alum mixed with ammonium alum to produce a purple
crystal
An interesting experiment would be grow a purple chrome alum seed crystal and
then grow a clear, transparent coating of ammonium alum around it. The two
types of alum are compatible and produce the same shape of crystal as shown in the next picture.

Purple alum crystal surrounded by a clear alum crystal
Chrome alum
is poisonous, so be careful!
Evaporation Technique
The evaporation technique is a slow process, but it produces satisfying clear
transparent sharp crystals. This process consists of two parts: first
growing seed crystals and then the main growing event.
The first step is to produce some seed crystals. If you are unsure of how
much alum to make, make two separate batches. Heat some water using an
electric drip coffee maker (don't use any coffee). Using disposable plastic
cups, place two heaping able spoons of powdered alum to the first cup and four
heaping tablespoons of powdered alum to the second cup. Add approximately
8 ounces of hot water to each cup, stir until all of the alum is dissolved.
Place cups were they will not be disturbed for at least one week.

Cup #1 had two heaping
tablespoons of Alum dissolved in 8 oz. of hot water.
Cup #2 had four heaping teaspoons of alum dissolved in 8 oz. of hot water.
The results shown are after 1 week of sitting and evaporating.
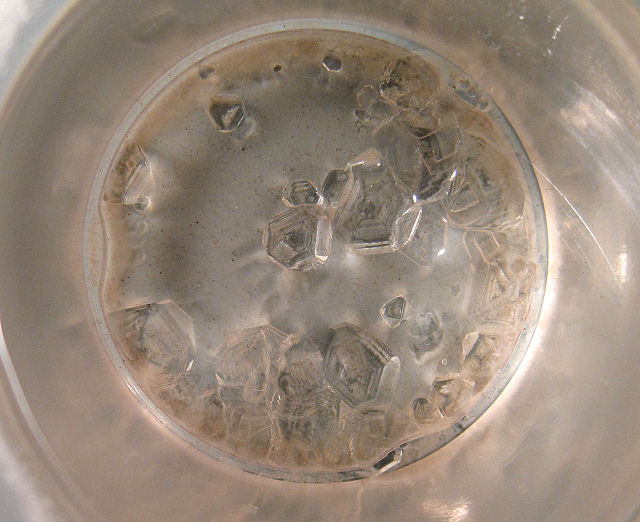
Cup #1 produced some nice
seed crystals.
The triangular shapes are the result of crystals lying on octahedral faces as
they grew. The cup was turned over on a paper towel and the crystals were dried.

Cup #2 had too much alum.
The crystals grew too rapidly are were clouded and overgrown.
Consequently, they were not used for seed crystals.

alum seed crystals from
cup #1, nice and clear.

Overlapping seed crystals
from cup #1.
The crystals need to be separated if you wish to grow a single nice crystal.

There are still two
individual intergrown alum crystals in this specimen.
They need to be separated before being placed in a growing solution.

Tie a thread lasso around
a single alum crystal.
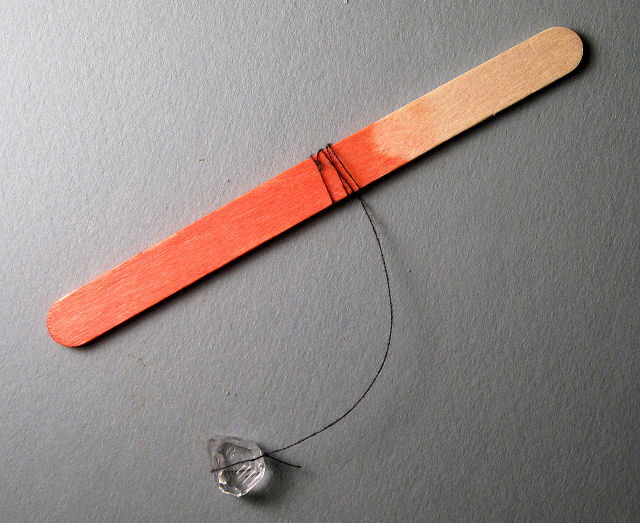
Tie the other end of the
thread around a popsicle stick.
Yes, this was a used popsicle stick; RECYCLE!

The saturated solution from
cup#1 was poured into a new clean cup, so that no additional seed crystals would start to grow. The prepared seed crystal
was then hung in the middle of the growing solution.
Place the solution where it can sit undisturbed and evaporate. The location
should be fairly dust-free because dust can trigger the development of
unwanted seed crystals.

Seed crystal is now covered by clear alum after 1 week
of evaporation.

A clear octahedron of
alum crystallized to an almost perfect octahedron form.
Food dye can be added to growing
solutions of alum in an attempt to add color to the crystals. However,
since crystallization is a purification process the growing alum crystals tend
to reject the food dye unless the dye is highly concentrated.
The following shows an attempt to add coloring to alum crystals by using food dye.

Too many seed crystals.

closer view

The crystals of alum were
distorted by growing so close together.

Here is an ammonium alum crystal that started growth while
lying on an octahedral face. The pink color is due to a small amount of chrome
alum added to the growing solution.